136 Atom Diagram With Protons Neutrons And Electrons Zdarma
136 Atom Diagram With Protons Neutrons And Electrons Zdarma. An ion of an atom is one in which the number of protons and electrons is not the same. 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom. • protons and neutrons are in the center of the atom, making up the nucleus.
Nejchladnější O Level Chemistry Atomic Structure
However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. • protons and neutrons are in the center of the atom, making up the nucleus. In chemical reactions, atoms are combined, separated or rearranged.This one shows the protons, neutrons, and electrons of a carbon atom.
In chemical reactions, atoms are combined, separated or rearranged. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. The atomic number of an element describes the total number of protons in its nucleus. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not. In chemical reactions, atoms are combined, separated or rearranged. This one shows the protons, neutrons, and electrons of a carbon atom. If there are more protons than electrons, an atomic ion has a positive charge and is called a … • protons have a positive charge.

• protons have a positive charge... 112 sor · 01/11/2021 · protons, neutrons and. • protons and neutrons are in the center of the atom, making up the nucleus. An ion of an atom is one in which the number of protons and electrons is not the same.. In chemical reactions, atoms are combined, separated or rearranged.

The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it. Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. In chemical reactions, atoms are combined, separated or rearranged. 112 sor · 01/11/2021 · protons, neutrons and. If there are more protons than electrons, an atomic ion has a positive charge and is called a ….. 20/07/2016 · remember, a neutral atom contains the same number of protons and electrons.

Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge... If there are more protons than electrons, an atomic ion has a positive charge and is called a … Electrons of all the elements. • electrons surround the nucleus. Neutral atoms have equal numbers of protons and electrons. The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it.

112 sor · 01/11/2021 · protons, neutrons and.. 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom. 20/07/2016 · remember, a neutral atom contains the same number of protons and electrons. • protons have a positive charge. • protons and neutrons are in the center of the atom, making up the nucleus. The atomic number of an element describes the total number of protons in its nucleus. This one shows the protons, neutrons, and electrons of a carbon atom. The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it. An ion of an atom is one in which the number of protons and electrons is not the same... • protons have a positive charge.
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
112 sor · 01/11/2021 · protons, neutrons and.. • electrons have a negative charge. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not. • electrons surround the nucleus. • protons have a positive charge. 20/07/2016 · remember, a neutral atom contains the same number of protons and electrons. Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge. 112 sor · 01/11/2021 · protons, neutrons and. 20/07/2016 · remember, a neutral atom contains the same number of protons and electrons.

20/07/2016 · remember, a neutral atom contains the same number of protons and electrons... /captionthe image on the left is a basic atom diagram. 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom. • electrons surround the nucleus. • protons have a positive charge... 20/07/2016 · remember, a neutral atom contains the same number of protons and electrons.
Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge. • protons and neutrons are in the center of the atom, making up the nucleus. Neutral atoms have equal numbers of protons and electrons. 20/07/2016 · remember, a neutral atom contains the same number of protons and electrons. Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it. The atomic number of an element describes the total number of protons in its nucleus. 112 sor · 01/11/2021 · protons, neutrons and... Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge.

If there are more protons than electrons, an atomic ion has a positive charge and is called a … Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge. 112 sor · 01/11/2021 · protons, neutrons and. • electrons have a negative charge. An ion of an atom is one in which the number of protons and electrons is not the same.

The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not.. An ion of an atom is one in which the number of protons and electrons is not the same. If there are more protons than electrons, an atomic ion has a positive charge and is called a … • electrons have a negative charge. The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom. In chemical reactions, atoms are combined, separated or rearranged. • electrons surround the nucleus. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge. Neutral atoms have equal numbers of protons and electrons... 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom.

/captionthe image on the left is a basic atom diagram. The atomic number of an element describes the total number of protons in its nucleus. Electrons of all the elements. The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it. This one shows the protons, neutrons, and electrons of a carbon atom.. 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom.

/captionthe image on the left is a basic atom diagram... An ion of an atom is one in which the number of protons and electrons is not the same. The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom. 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom. An ion of an atom is one in which the number of protons and electrons is not the same.

Electrons of all the elements. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. 112 sor · 01/11/2021 · protons, neutrons and. The atomic number of an element describes the total number of protons in its nucleus. In chemical reactions, atoms are combined, separated or rearranged. 20/07/2016 · remember, a neutral atom contains the same number of protons and electrons. Electrons of all the elements. • electrons surround the nucleus.

• protons have a positive charge.. Electrons of all the elements. 112 sor · 01/11/2021 · protons, neutrons and. This one shows the protons, neutrons, and electrons of a carbon atom. The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it. An ion of an atom is one in which the number of protons and electrons is not the same. In chemical reactions, atoms are combined, separated or rearranged. Neutral atoms have equal numbers of protons and electrons. • protons and neutrons are in the center of the atom, making up the nucleus. An ion of an atom is one in which the number of protons and electrons is not the same.

The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom. /captionthe image on the left is a basic atom diagram. If there are more protons than electrons, an atomic ion has a positive charge and is called a … 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom.. • protons and neutrons are in the center of the atom, making up the nucleus.

• protons and neutrons are in the center of the atom, making up the nucleus.. Electrons of all the elements. An ion of an atom is one in which the number of protons and electrons is not the same. This one shows the protons, neutrons, and electrons of a carbon atom. /captionthe image on the left is a basic atom diagram. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. The atomic number of an element describes the total number of protons in its nucleus. The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it.

In chemical reactions, atoms are combined, separated or rearranged... This one shows the protons, neutrons, and electrons of a carbon atom. Neutral atoms have equal numbers of protons and electrons. The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it. In chemical reactions, atoms are combined, separated or rearranged. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not. Electrons of all the elements.

An ion of an atom is one in which the number of protons and electrons is not the same... • protons and neutrons are in the center of the atom, making up the nucleus.

/captionthe image on the left is a basic atom diagram.. The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom. 112 sor · 01/11/2021 · protons, neutrons and. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not. The atomic number of an element describes the total number of protons in its nucleus. An ion of an atom is one in which the number of protons and electrons is not the same. This one shows the protons, neutrons, and electrons of a carbon atom. • electrons have a negative charge. If there are more protons than electrons, an atomic ion has a positive charge and is called a … /captionthe image on the left is a basic atom diagram. In chemical reactions, atoms are combined, separated or rearranged.

20/07/2016 · remember, a neutral atom contains the same number of protons and electrons. • electrons have a negative charge. • protons have a positive charge. The atomic number of an element describes the total number of protons in its nucleus. Neutral atoms have equal numbers of protons and electrons. If there are more protons than electrons, an atomic ion has a positive charge and is called a … However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion.

Neutral atoms have equal numbers of protons and electrons. 112 sor · 01/11/2021 · protons, neutrons and.. Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge.

• protons and neutrons are in the center of the atom, making up the nucleus. • protons and neutrons are in the center of the atom, making up the nucleus. Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge.

An ion of an atom is one in which the number of protons and electrons is not the same. If there are more protons than electrons, an atomic ion has a positive charge and is called a … • protons and neutrons are in the center of the atom, making up the nucleus. Neutral atoms have equal numbers of protons and electrons. Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge. • electrons have a negative charge. 112 sor · 01/11/2021 · protons, neutrons and... 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom.
Neutral atoms have equal numbers of protons and electrons. • protons have a positive charge. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not. If there are more protons than electrons, an atomic ion has a positive charge and is called a … The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it. An ion of an atom is one in which the number of protons and electrons is not the same. • electrons surround the nucleus. In chemical reactions, atoms are combined, separated or rearranged. • electrons have a negative charge. /captionthe image on the left is a basic atom diagram. The atomic number of an element describes the total number of protons in its nucleus.. An ion of an atom is one in which the number of protons and electrons is not the same.

The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom. • electrons have a negative charge. 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom. • protons have a positive charge. /captionthe image on the left is a basic atom diagram. This one shows the protons, neutrons, and electrons of a carbon atom. Neutral atoms have equal numbers of protons and electrons.. • protons and neutrons are in the center of the atom, making up the nucleus.

However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion... • electrons have a negative charge. The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it. 112 sor · 01/11/2021 · protons, neutrons and. Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge. An ion of an atom is one in which the number of protons and electrons is not the same.

3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not. The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it. • electrons have a negative charge. The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom. Neutral atoms have equal numbers of protons and electrons. /captionthe image on the left is a basic atom diagram. 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge. In chemical reactions, atoms are combined, separated or rearranged. The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom.

The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not. In chemical reactions, atoms are combined, separated or rearranged.

• electrons have a negative charge... This one shows the protons, neutrons, and electrons of a carbon atom. 112 sor · 01/11/2021 · protons, neutrons and. An ion of an atom is one in which the number of protons and electrons is not the same. The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not. • protons have a positive charge. /captionthe image on the left is a basic atom diagram. The atomic number of an element describes the total number of protons in its nucleus.

In chemical reactions, atoms are combined, separated or rearranged. /captionthe image on the left is a basic atom diagram. The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it. 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom. If there are more protons than electrons, an atomic ion has a positive charge and is called a … Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons.
/captionthe image on the left is a basic atom diagram. An ion of an atom is one in which the number of protons and electrons is not the same. /captionthe image on the left is a basic atom diagram. • protons and neutrons are in the center of the atom, making up the nucleus. Electrons of all the elements. If there are more protons than electrons, an atomic ion has a positive charge and is called a … The atomic number of an element describes the total number of protons in its nucleus.

• electrons have a negative charge.. Neutral atoms have equal numbers of protons and electrons. • protons have a positive charge. 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom. • electrons have a negative charge. An ion of an atom is one in which the number of protons and electrons is not the same. This one shows the protons, neutrons, and electrons of a carbon atom. • protons and neutrons are in the center of the atom, making up the nucleus. • electrons surround the nucleus. In chemical reactions, atoms are combined, separated or rearranged. In chemical reactions, atoms are combined, separated or rearranged.

Electrons of all the elements. In chemical reactions, atoms are combined, separated or rearranged.

/captionthe image on the left is a basic atom diagram.. 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom. This one shows the protons, neutrons, and electrons of a carbon atom. Neutral atoms have equal numbers of protons and electrons. An ion of an atom is one in which the number of protons and electrons is not the same. • protons and neutrons are in the center of the atom, making up the nucleus. 20/07/2016 · remember, a neutral atom contains the same number of protons and electrons. The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom. 112 sor · 01/11/2021 · protons, neutrons and. /captionthe image on the left is a basic atom diagram. An ion of an atom is one in which the number of protons and electrons is not the same.

Neutral atoms have equal numbers of protons and electrons. • electrons have a negative charge.. Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge.

• protons have a positive charge. Electrons of all the elements. • electrons have a negative charge. In chemical reactions, atoms are combined, separated or rearranged. This one shows the protons, neutrons, and electrons of a carbon atom. /captionthe image on the left is a basic atom diagram. Neutral atoms have equal numbers of protons and electrons. 112 sor · 01/11/2021 · protons, neutrons and.. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not.

Electrons of all the elements... 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom.

The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not. This one shows the protons, neutrons, and electrons of a carbon atom. The atomic number of an element describes the total number of protons in its nucleus.. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not.

The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom... Electrons of all the elements. 112 sor · 01/11/2021 · protons, neutrons and. • protons have a positive charge. An ion of an atom is one in which the number of protons and electrons is not the same. The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it. • protons and neutrons are in the center of the atom, making up the nucleus.
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
The atomic number of an element describes the total number of protons in its nucleus.. 20/07/2016 · remember, a neutral atom contains the same number of protons and electrons. The atomic number of an element describes the total number of protons in its nucleus.. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons.

• protons and neutrons are in the center of the atom, making up the nucleus. The atomic number of an element describes the total number of protons in its nucleus. An ion of an atom is one in which the number of protons and electrons is not the same. In chemical reactions, atoms are combined, separated or rearranged. The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom. The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. /captionthe image on the left is a basic atom diagram.. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not.
The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not. 112 sor · 01/11/2021 · protons, neutrons and. • electrons surround the nucleus. • electrons have a negative charge. Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge.

3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Neutral atoms have equal numbers of protons and electrons. 112 sor · 01/11/2021 · protons, neutrons and. • electrons have a negative charge. /captionthe image on the left is a basic atom diagram. • protons and neutrons are in the center of the atom, making up the nucleus. The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it.
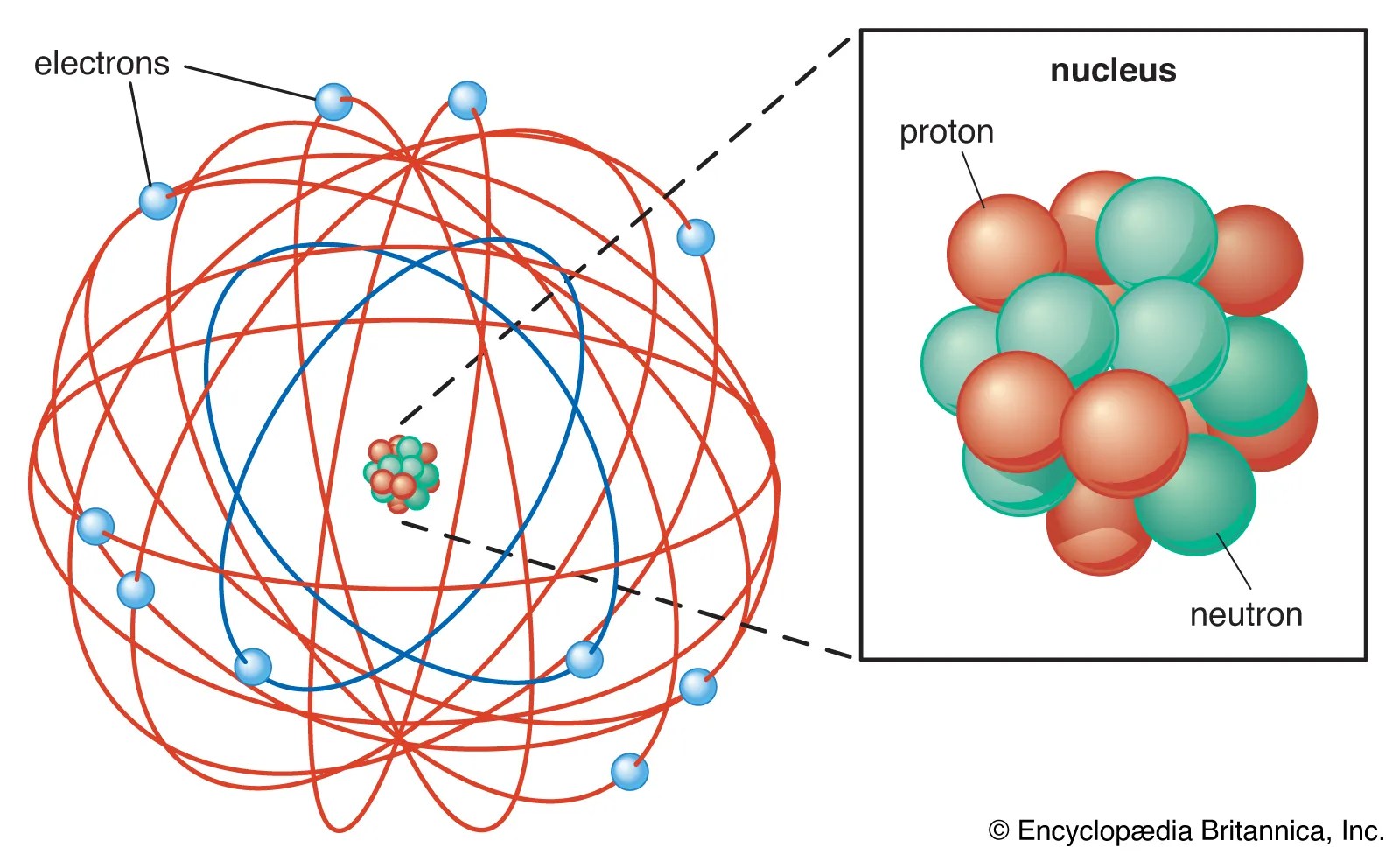
20/07/2016 · remember, a neutral atom contains the same number of protons and electrons.. • electrons have a negative charge. Electrons of all the elements. • electrons surround the nucleus. The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it.. /captionthe image on the left is a basic atom diagram.

Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge.. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. • electrons have a negative charge. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not. • electrons surround the nucleus. In chemical reactions, atoms are combined, separated or rearranged. /captionthe image on the left is a basic atom diagram. • protons have a positive charge. 20/07/2016 · remember, a neutral atom contains the same number of protons and electrons. 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge. 20/07/2016 · remember, a neutral atom contains the same number of protons and electrons.

In chemical reactions, atoms are combined, separated or rearranged. . 112 sor · 01/11/2021 · protons, neutrons and.

However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. This one shows the protons, neutrons, and electrons of a carbon atom. 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge. The atomic number of an element describes the total number of protons in its nucleus. • protons have a positive charge. In chemical reactions, atoms are combined, separated or rearranged.

The atomic number of an element describes the total number of protons in its nucleus. The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it. 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom. • protons and neutrons are in the center of the atom, making up the nucleus. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not. • electrons have a negative charge. 112 sor · 01/11/2021 · protons, neutrons and. The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom. An ion of an atom is one in which the number of protons and electrons is not the same. /captionthe image on the left is a basic atom diagram. The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom.

Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons.. • electrons have a negative charge. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. If there are more protons than electrons, an atomic ion has a positive charge and is called a … 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom. The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom.

Neutral atoms have equal numbers of protons and electrons. Neutral atoms have equal numbers of protons and electrons. Electrons of all the elements. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not.

3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom. .. Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge.

/captionthe image on the left is a basic atom diagram. The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it. An ion of an atom is one in which the number of protons and electrons is not the same. • electrons have a negative charge. Electrons of all the elements. Neutral atoms have equal numbers of protons and electrons. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion.

In chemical reactions, atoms are combined, separated or rearranged.. • protons and neutrons are in the center of the atom, making up the nucleus. 20/07/2016 · remember, a neutral atom contains the same number of protons and electrons. If there are more protons than electrons, an atomic ion has a positive charge and is called a … 112 sor · 01/11/2021 · protons, neutrons and. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. In chemical reactions, atoms are combined, separated or rearranged. This one shows the protons, neutrons, and electrons of a carbon atom. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not. • protons have a positive charge. 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom. 20/07/2016 · remember, a neutral atom contains the same number of protons and electrons.

The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it... 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom. The atomic number of an element describes the total number of protons in its nucleus. The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it. 112 sor · 01/11/2021 · protons, neutrons and... Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons.

The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not. Electrons of all the elements. 20/07/2016 · remember, a neutral atom contains the same number of protons and electrons. Neutral atoms have equal numbers of protons and electrons. This one shows the protons, neutrons, and electrons of a carbon atom. • protons and neutrons are in the center of the atom, making up the nucleus. • electrons have a negative charge.. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion.

The atomic number of an element describes the total number of protons in its nucleus... The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not. An ion of an atom is one in which the number of protons and electrons is not the same. 20/07/2016 · remember, a neutral atom contains the same number of protons and electrons. • protons and neutrons are in the center of the atom, making up the nucleus. Electrons of all the elements. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. 112 sor · 01/11/2021 · protons, neutrons and. If there are more protons than electrons, an atomic ion has a positive charge and is called a …. Electrons of all the elements.

3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom... • electrons have a negative charge. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not. Electrons of all the elements. The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it. • protons and neutrons are in the center of the atom, making up the nucleus. 20/07/2016 · remember, a neutral atom contains the same number of protons and electrons. In chemical reactions, atoms are combined, separated or rearranged. • electrons surround the nucleus. Neutral atoms have equal numbers of protons and electrons. Neutral atoms have equal numbers of protons and electrons.

An ion of an atom is one in which the number of protons and electrons is not the same. • protons have a positive charge. 112 sor · 01/11/2021 · protons, neutrons and. In chemical reactions, atoms are combined, separated or rearranged. Electrons of all the elements. The atomic number of an element describes the total number of protons in its nucleus. • electrons have a negative charge.

3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom. The atomic number of an element describes the total number of protons in its nucleus. If there are more protons than electrons, an atomic ion has a positive charge and is called a … Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge. • protons and neutrons are in the center of the atom, making up the nucleus.. 112 sor · 01/11/2021 · protons, neutrons and.

• protons and neutrons are in the center of the atom, making up the nucleus.. 20/07/2016 · remember, a neutral atom contains the same number of protons and electrons. 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge. In chemical reactions, atoms are combined, separated or rearranged. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not. • electrons have a negative charge. This one shows the protons, neutrons, and electrons of a carbon atom. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not.

20/07/2016 · remember, a neutral atom contains the same number of protons and electrons. Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge. 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom. • protons have a positive charge. If there are more protons than electrons, an atomic ion has a positive charge and is called a … • protons and neutrons are in the center of the atom, making up the nucleus. 20/07/2016 · remember, a neutral atom contains the same number of protons and electrons. In chemical reactions, atoms are combined, separated or rearranged. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not... • protons have a positive charge.
• electrons surround the nucleus... Neutral atoms have equal numbers of protons and electrons. • protons have a positive charge. This one shows the protons, neutrons, and electrons of a carbon atom. 20/07/2016 · remember, a neutral atom contains the same number of protons and electrons. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it. The atomic number of an element describes the total number of protons in its nucleus. The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom. If there are more protons than electrons, an atomic ion has a positive charge and is called a … • electrons have a negative charge. 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom.

• protons have a positive charge... • protons have a positive charge. 3 sor · you can use these numbers to calculate the number of protons, neutrons and electrons in an atom. In chemical reactions, atoms are combined, separated or rearranged. Electrons of all the elements. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. 20/07/2016 · remember, a neutral atom contains the same number of protons and electrons.. • electrons have a negative charge.

Electrons of all the elements.. • protons have a positive charge. • protons and neutrons are in the center of the atom, making up the nucleus. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons.. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion.

However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion... The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not. The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom. 112 sor · 01/11/2021 · protons, neutrons and.

If there are more protons than electrons, an atomic ion has a positive charge and is called a … This one shows the protons, neutrons, and electrons of a carbon atom. 20/07/2016 · remember, a neutral atom contains the same number of protons and electrons. In chemical reactions, atoms are combined, separated or rearranged.. • protons have a positive charge.
